Membrane protein structure and assembly
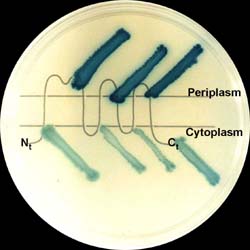 Since membrane proteins are particularly difficult to crystallize and, thus, difficult to structurally analyze, we have developed genetic techniques to determine aspects of their structure. First, we presented a gene fusion technique that allows one to describe the topology of a membrane protein. This approach generates a model for the arrangement of the protein in the membrane including which hydrophilic domains are in the cytoplasm and which are in the extracytoplasmic space (see picture).We have also developed a reporter system that permits detection of interaction between transmembrane segments of proteins. For instance, it can detect those transmembrane segments that are capable of homodimerization. We are also interested in the mechanism of assembly of proteins into membranes. A genetic screeing procedure for defects in membrane protein insertion has revealed mutants in a bacterial homologue of a component of the eukaryotic signal recognition particle and we are analyzing them using a sensitive reporter system. Finally, we have developed a computer program that allows one to take sequences of genomes of organisms and estimate how many membrane proteins that organism is capable of encoding. |
|
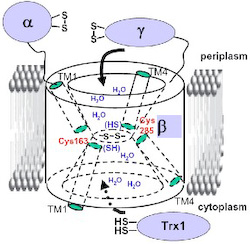
Disulfide bond isomerization in bacterial periplasm is mediated by DsbC. The reduced state of DsbC is necessary for its activity and maintained by DsbD. DsbD consists of two periplasmic domains and a membrane-embedded one, and transfers electrons from the cytoplasm to the periplasm. The transmembrane domain interacts with the cytoplasmic thioredoxin-1 and its periplasmic thioredoxin-fold domain, catalyzing electron transfer between them. By structural and functional study, we showed that the two redox-active cysteines of transmembrane domain are exposed to both compartments and the structure of it shows an hourglass-like shape. We are further characterizing the structural features of this unusual transmembrane domain. Especially, we are trying to get high-resolution structure of DsbD or a homologue.
|
|
